There is a lot of attention being paid to new, improved battery chemistries and construction, for well-known reasons. After all, even a small percentage improvement in battery performance by volume or weight can have significant impact in the big picture of energy storage and supply power as needed. Still, researchers are looking for the big breakthrough, and while there have been claims of smallish-to-medium ones, going from a lab version of an improved battery to even a modest pilot run is very different than true mass production with millions of units and long-term performance issues.
There’s another or side to the battery equation that is really important: recycling of discharged primary (non-rechargeable) cells and exhausted secondary (rechargeable) cells. Both are major consumers of minerals and other materials, of course, but due to the growth of electric vehicles (EVs) of all types and energy-storage systems (ESSs), the rechargeable Li-ion batteries are getting the most attention. The reality is that there is no single loop for battery recycling (Figure 1).
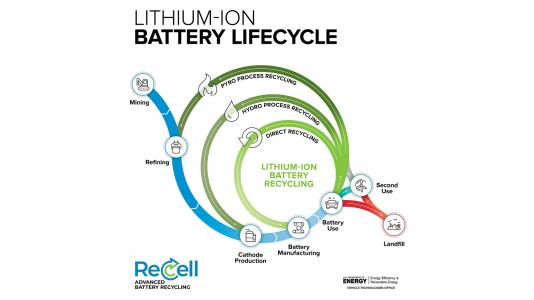
What fraction of primary and secondary batteries are recycled now? As with so many of these questions, the answer depends on both the context of the question and the source of the data. For example:
• Do you measure by the number of batteries, their weight or energy capacity?
• Do you include small button and coin-cell batteries that are usually tossed in the trash (even though they shouldn’t be), mid-size packs from laptops and smart phones, as well as larger battery assemblies in EVs and ESSs?
• Do you use country, regional, or worldwide numbers?
• How does the number or weight of batteries sent to recycling relate to the amount of material that is actually recovered and reusable?
• How do the number vary among battery chemistries?
• And finally, the ever-present statistical data-credibility question: Does the apparent precision often given with these numbers also have corresponding accuracy?
Research on these matters shows that numbers are all over the place. Among the ones that seemed to have some consistency and credibility were that at least 95% of the classic-and-still-widely-used lead-acid batteries were recycled, while only about 5% of the lithium-based batteries (“other”) are recycled, (Figure 2) in the European Union; U.S. numbers were about the same. (The research presented here is from sources that do not require an annual subscription for access.)
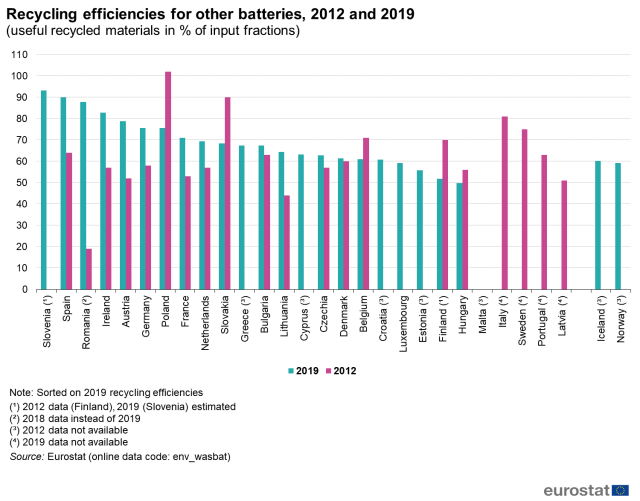
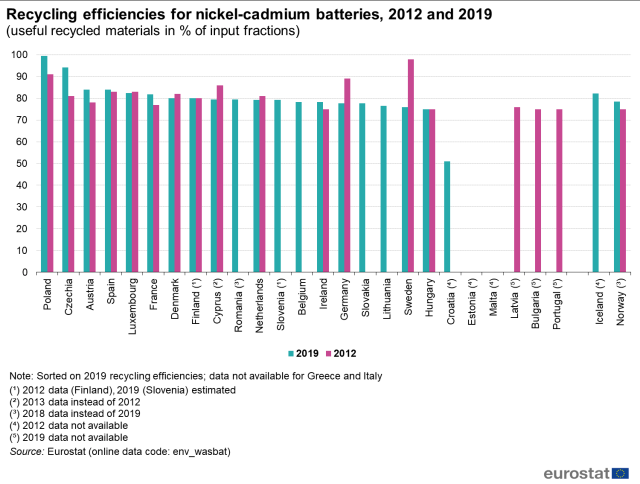

Why the difference in recycling versus battery type? There are several reasons.
First, recycling and reclaiming the lead in a battery is technically far simpler than doing the same for the complex amalgamation of minerals such as cobalt, manganese, phosphates, and lithium in what we call “lithium” batteries.
Also, lead-acid batteries come in relatively few physical form factors.
Finally, there’s a well-developed recycle chain for these batteries, which are often removed at car dealers, auto-supply stores or scrap yards.
Nonetheless, the potential benefits of recycling batteries and reclaiming/reusing constituent materials are attracting a lot of interest and that translates to money, with investments to improve the basic technologies, as well as to build facilities to do this. The Wall Street Journal recently reported on the wave of money (some government grants, some private investment) being directed at recycling research and large plants. Some of these plants are independent operations who will then resell reclaimed materials to whoever they can, while others are aligning themselves formally or informally with battery makers or car manufacturers.
The problem they all face is a consequence of modern technology, and it goes beyond just batteries.
What makes many of our achievements possible is that we have learned, via materials science, how to modify, combine, blend and adapt basic substances (stone, wood, minerals, elements, oil, gas, and ores) to create materials with just the right properties needed to provide needed performance across multiple attributes. (Think of “simple” connectors with their glass-epoxy bodies, metal-alloy contacts, and thin surface platings of various types.) So instead of basic, easily defined materials in a product or device, you have a complex combination of all modified and merged.
Disaggregating these materials to restore them to their original and thus reusable constituents is difficult and sometimes impossible. It is not just a matter of unscrewing, ungluing, or unnailing tangible pieces but instead requires significant energy, chemicals, physical space and more, and often produces nasty residuals as well. In colloquial terms, it’s tough unscramble that egg that unless you apply a lot of technology and energy.
Perhaps it’s yet another manifestation of the principle of entropy with a tendency toward universal disorder, so trying to re-establish order is a large if not impossible task. Think of the random mixing of gases when they released into a chamber
Does practical and effective battery recycling and subsequent reclaiming of material need a breakthrough? (For example, Argonne National Laboratory claims a “breakthrough” for lithium batteries, but that’s a big claim to make at this early stage, see Reference 1). Or is it just a case of slow and steady progress will get us there? Will it only become viable when scarcity and cost of mining and refining new minerals and materials becomes much more burdensome compared to reclaiming and re-using existing products? What’s the role of the entire recycling chain in ensuring a steady supply or pure materials?
Just wanting it to be so won’t necessarily make it happen, despite money and best intentions. Recycling, recovering, and reusing these advanced formulations is far harder than doing the same for simpler products such as paper, for example, and even that is very complicated. Stayed tuned, it’s an interesting ride ahead.
References
Argonne National Laboratory, “Breakthrough research makes battery recycling more economical”This article was originally published on EE Times.
Bill Schweber is an electronics engineer who has written three textbooks on electronic communications systems, as well as hundreds of technical articles, opinion columns, and product features. In past roles, he worked as a technical website manager for multiple EE Times sites and as both Executive Editor and Analog Editor at EDN. At Analog Devices, he was in marketing communications; as a result, he has been on both sides of the technical PR function, presenting company products, stories, and messages to the media and also as the recipient of these. Prior to the marcom role at Analog, Bill was Associate Editor of its respected technical journal, and also worked in its product marketing and applications engineering groups. Before those roles, he was at Instron Corp., doing hands-on analog- and power-circuit design and systems integration for materials-testing machine controls. He has a BSEE from Columbia University and an MSEE from the University of Massachusetts, is a Registered Professional Engineer, and holds an Advanced Class amateur radio license. He has also planned, written, and presented online courses on a variety of engineering topics, including MOSFET basics, ADC selection, and driving LEDs.
